D-Dimer Rapid Test


D-Dimer Rapid Test
D-Dimer Rapid test is an immunoassay designed for the quantitative determination of D-Dimer concentration.
This test can be used as an aid in the assessment and evaluation of suspected DVT and PE patients.
D-Dimer test is performed when there is a suspicion of deep venous thrombosis (DVT) or pulmonary embolism (PE).
Features
Features & Functions
This test can be used as an aid in the assessment and evaluation of suspected DVT and PE patients.
D-Dimer rapid diagnostic test is performed when there is a suspicion of deep venous thrombosis (DVT) or pulmonary embolism (PE).
- A negative result practically rules out thrombosis, a positive result can indicate thrombosis but does not rule out other potential aetiologies.
- The main use is to exclude thromboembolic disease where the probability is low.
- In patients suspected of disseminated intravascular coagulation, D- Dimer testing may aid in the diagnosis.
- D-Dimer for DVT / PE : Adult cut off for exclusion of VTE: < 500 ng/mL [0.5mg/L] to help rule out pulmonary embolism
| Samples: | Plasma or whole blood |
| Anticoagulant: | Sodium citrate |
| Sample Volume: | 120uL |
| Reaction Time: | 7 minutes |
| Storage: | 4 to 30°C |
| Shelf life: | up to 24 months |
Performance Characteristics
Key Performance Statistics
| Sensitivity | 87.0% |
| Specificity | 93.55% |
| Positive Predictive Value (PPV) | 93.1% |
| Negative Predictive Value (NPV) | 87.88% |
| Limit of Detection Measuring Range |
0.2mg/L [190ng/L] 0.2 – 10mg/L [200 – 10,000ng/l] |
| Linear Range [CLSI EPA-6] | 0.4 – 8.0 mg/L [400- 8,000ng/l] |
Clinical Performance
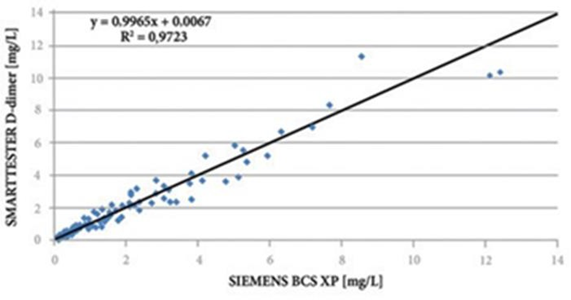
The D-Dimer concentration of samples was determined by Siemens BCS XP according to the CLSI-EP09-A3 guideline, with a cut off concentration of 0.5 mg/L. A total of 120 samples of whole blood and plasma/serum, respectively, with D-dimer level of 0.17-34.25 mg/L were used. The results from the linear regression analysis of the comparison data are shown below.
| Test Result Siemens SmartTester | Subject Affected | Subject Unaffected |
| Positive | 27 | 2 |
| Negative | 4 | 29 |
[3] Clinical and Laboratory Standards Institute, “Evaluation of the Linearity of Quantitative Measurement Procedures: A Statistical Approach, Approved Guideline. NCCLS Document EP6-A,” NCCLS Doc. EP6-A, vol. 23, no. 16, pp. 1–50, 2003.
[4] CLSI, EP09-A3 Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline—Third Edition This, no. August. 2013.
Precision
Performance claim: < 15%
Intra-assay
Results from 5 devices, 3 lots, 3 concentrations
| 0.8 mg/L - 9.32% | [800ng/L] |
| 2.01mg/L - 6.23% | [2010ng/L] |
| 2.96 mg /L - 8.46% | [2960ng/L] |
Inter-assay
D-dimer 2.0mg/L control 10 test cards of three lots
Intra-assay precision SD=0.186mg/L
Intra Assay precision = SD/r = 9.25%
Variants and Related Products
Variants and Related Products
| Order Code | Contents | Size |
| STR-9911 | SmartTester Reader | 1 device |
| RTC-9901-1 | CRP Rapid Test cartridges | 25 tests |
| RTC-9913-1 | hCG Rapid Test Cartridges | 25 tests |
| RTC-9902-1 | D-Dimer Rapid Test Cartridges | 25 tests |
| RTC-9911-1 | Microalbumin Rapid Test Cartridges | 25 tests |
| RTC-9912-1 | TSH Rapid Test Cartridges | 25 tests |
| RTC-9903-1 | Cardiac Troponin l (cTnI) Rapid Test cartridges | 25 tests |
